
Most of the representative metals do not occur naturally in an uncombined state because they readily react with water and oxygen in the air.
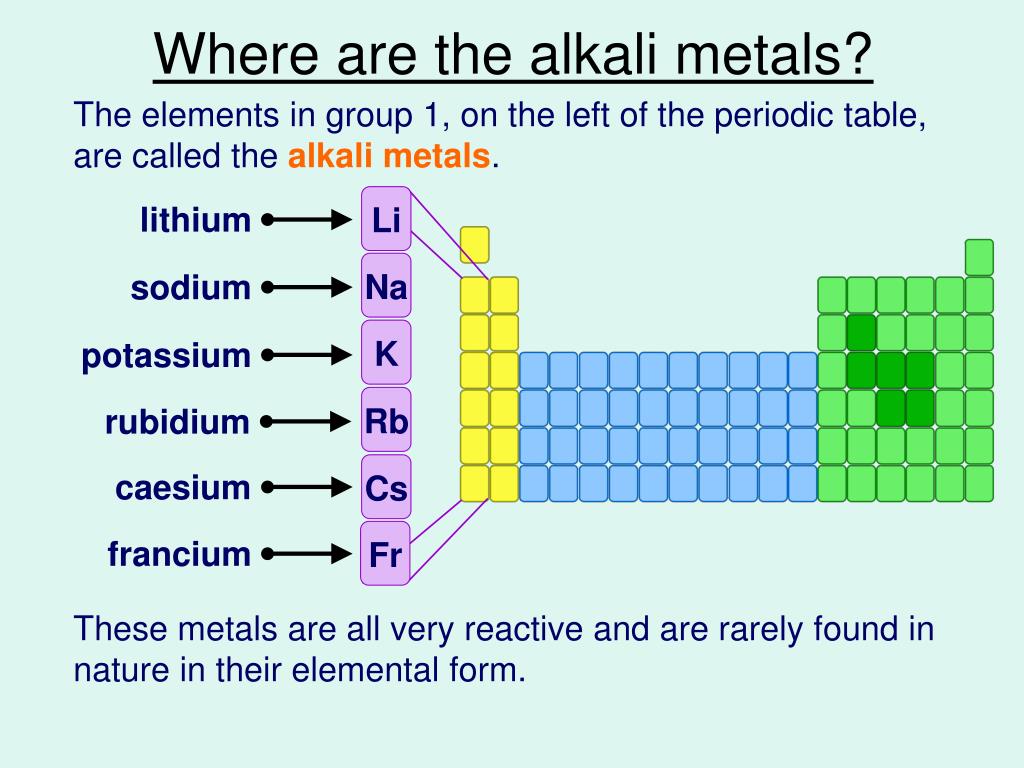
Nonmetals are shown in green, metalloids in purple, and the transition metals and inner transition metals in blue. A salt is an ionic compound consisting of cations and anions.įigure 18.2 The location of the representative metals is shown in the periodic table. In general, the combination of a metal and a nonmetal produces a salt. Unlike metals, which typically form cations and ionic compounds (containing ionic bonds), nonmetals tend to form anions or molecular compounds. The remaining representative elements are nonmetals. A metalloid is an element that has properties that are between those of metals and nonmetals these elements are typically semiconductors. In addition to the representative metals, some of the representative elements are metalloids. The radioactive elements copernicium, flerovium, polonium, and livermorium are also metals but are beyond the scope of this chapter. There are 20 nonradioactive representative metals in groups 1, 2, 3, 12, 13, 14, and 15 of the periodic table (the elements shaded in yellow in Figure 18.2). Metallic character results from an element’s ability to lose its outer valence electrons and results in high thermal and electrical conductivity, among other physical and chemical properties. Metals among the representative elements are the representative metals. The d orbitals fill with the elements in group 11 therefore, the elements in group 12 qualify as representative elements because the last electron enters an s orbital. The transition elements are elements where the d orbitals (groups 3–11 on the periodic table) are filling, and the inner transition metals are the elements where the f orbitals are filling. The representative elements are elements where the s and p orbitals are filling. It is possible to divide elements into groups according to their electron configurations.

The primary focus of this section will be the application of periodicity to the representative metals. We begin this section by examining the behaviors of representative metals in relation to their positions in the periodic table.


 0 kommentar(er)
0 kommentar(er)
